Why focus on inflammatory conditions?
At Wellfleet, we are always looking to improve access to clinically appropriate treatments, while also lowering costs for our plans and student members. Continuous analysis of the safety, effectiveness, and cost of the drugs covered by our Wellfleet Rx Student Formulary is part of that approach.
To that end, we turned our focus to an analysis of the impacts associated with quantity limits and prior authorization utilization management strategies applied to Inflammatory Condition (IC) medications between August 2021 – July 2022. Our attention shifted to this class because of the importance of those medications for our student members, as well as the financial impacts on them and their school plans.
In fact, for Wellfleet Rx, the Inflammatory Condition drug class is the largest category of medication spend, accounting for more than one quarter (28%) of overall plan spend. One familiar drug that has a big impact in this category is the Humira (CF) Pen, which accounts for 34% of the total spend in the Inflammatory Condition class.1
With respect to utilization management programs, they help ensure student members are receiving the most appropriate medications while helping to lower the overall cost of health care. To learn more, check out our article on how Wellfleet Rx utilization management strategies help students and schools win.
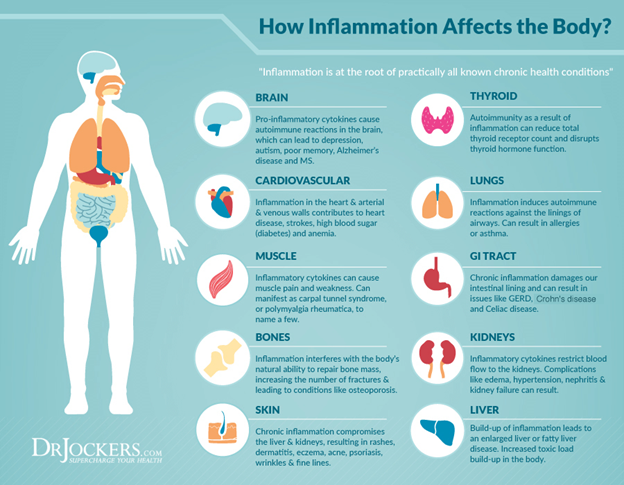
What is an inflammatory condition?
Inflammation is a normal, biological reaction to stimuli that the body interprets as potentially harmful. In its simplest form, think of getting a splinter in your finger. In addition to some pain, the area around the splinter gets hot and red. This heat and color are due to the immune system attacking the foreign object and contaminants.
With an inflammatory condition, the immune system malfunctions, causing inflammation that incorrectly targets one’s own cells or tissues.2 While this can occur in a variety of ways like with Ulcerative colitis or Rheumatoid arthritis, inflammatory responses often result in chronic pain, redness, swelling, stiffness, and damage to otherwise healthy body tissues.2
Member savings from inflammatory condition utilization management
When reviewing the total student population impacted by conditions that require Inflammatory Condition medications, like Humira, there were several requests for Prior Authorization and Quantity Limit approvals. While many requests were approved for the originally requested medication and quantity (73%), others were guided to alternative medications or therapies.1
When calculating the member savings generated, it resulted in $1.13 PMPM (per member, per month)!1
Lower cost alternatives coming soon
While inflammatory condition medications help improve the quality of life of the utilizers, they are commonly the highest spend category for insurance plans as the majority of them are high-cost, brand-name injectables.3
In the near future, patent protection on these brand names will expire, helping drive down consumer costs.
Also, Biosimilar products, those that are highly similar to existing FDA-approved products,4 are being released within the next four years.5 This is great news, as biosimilar products typically have a lower price tag compared to branded products.
In 2023, a number of interchangeable biosimilar therapies for inflammatory conditions are expected to come to market driving cost competition in this therapy class.5 Of note, biosimilar products for Humira will begin to go to market in February 2023, and biosimilar products for Stelara will begin to go to market in Q4 of 2023.6
As these products become available, Wellfleet will review and update our formulary as appropriate. Want to learn more? Click here to find out how biosimilars will alter the current pharmaceutical landscape.
Resources
1 Wellfleet (2022, December 16). Inflammatory conditions outcomes. A detailed analysis of plan year 2021-2022. Internal Wellfleet document not published.
2 Cleveland Clinic. (n.d.). Inflammation. Retrieved on January 6, 2023, from https://my.clevelandclinic.org/health/symptoms/21660-inflammation.
3 Kamal, R., Cox, C., McDermott, D. (2019, February 20). What are the recent and forecasted trends in prescription drug spending? https://www.healthsystemtracker.org/chart-collection/recent-forecasted-trends-prescription-drug-spending/#Chart
4 FDA.gov. (2022, December 13) Biosimilars Review and approval. Retrieved on January 6, 2023, from https://www.fda.gov/drugs/biosimilars/review-and-approval.
5 Zamecnik, A. (2022, December 5). Humira biosimilars set the stage for long-awaited 2023 US launches. Retrieved on January 6, 2023, from https://www.pharmaceutical-technology.com/features/humira-biosimilars-set-the-stage-for-long-awaited-2023-us-launches/
6 Stewart, J. (2022, December 23). How many biosimilars have been approved in the United States? Retrieved on January 6, 2023, from https://www.drugs.com/medical-answers/many-biosimilars-approved-united-states-3463281/.


